A) 32.
B) 30.
C) 24.
D) 28.
E) 26.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
All of the following have ground-state noble-gas electron configurations except
A) Ar
B) N3-
C) P3+
D) Mg2+
E) Cl-
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following Lewis structures best describes BF3?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following ions in order of decreasing atomic radii: Te2-,Te4+,Te6+.
A) Te2- > Te4+ > Te6+
B) Te6+ > Te4+ > Te2-
C) Te4+ > Te2- > Te6+
D) Te2- > Te6+ > Te4+
E) Te4+ > Te6+ > Te2-
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a correct Lewis electron-dot formula for CO?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The number of valence electrons in the propionate ion,CH3CH2COO-,is
A) 30.
B) 32.
C) 36.
D) 50.
E) 28.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
All of the following species are isoelectronic except
A) S2-
B) K+
C) Na+
D) Ar
E) Cl-
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following has an enthalpy change that is equal to the lattice energy of SrCl2?
A) SrCl2(s) ? Sr(g) + 2Cl(g)
B) SrCl2(s) ? Sr(g) + Cl2(g)
C) SrCl2(s) ? Sr2+(g) + 2Cl(g)
D) SrCl2(s) ? Sr(s) + Cl2(g)
E) SrCl2(s) ? Sr2+(g) + 2Cl-(g)
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many valence electrons are there in the trifluoroacetate ion,CF3COO-?
A) 41
B) 42
C) 54
D) 56
E) 40
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following processes is not exothermic?
A) Rb+(g) + e- → Rb(g)
B) Rb+(g) + Cl-(g) → RbCl(s)
C) Cl(g) + e- → Cl-(g)
D) Rb(g) → Rb(s)
E) ![]() Cl2(g) → Cl(g)
Cl2(g) → Cl(g)
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For each of the following species except ____,the electronic structure may be adequately described by two resonance formulas.
A) O3
B) SO32-
C) NO2-
D) C6H6
E) SO2
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using bond-energy data,what is ΔH° for the following reaction? CH3OH(g) + H2S(g) → CH3SH(g) + H2O(g) Bond Bond Energy (kJ/mol) C-H 413 C-O 358 O-H 463 C-S 259 S-H 339
A) -25 kJ
B) -124 kJ
C) 25 kJ
D) -2763 kJ
E) 2738 kJ
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a correct description of lattice energy?
A) The energy change that occurs when electrons are removed from a lattice.
B) The energy change that occurs when a gas condenses to a liquid.
C) The energy change that occurs when a liquid freezes.
D) The energy change that occurs when an ionic solid is separated into its ions in the gas phase.
E) The lattice energy of a substance is identical to the ionic bond energy determined from coulombs law.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many valence electrons does a carbonate ion have?
A) 30
B) 28
C) 24
D) 32
E) 22
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the total number of valence electrons in N2O?
A) 16
B) 17
C) 34
D) 11
E) 22
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following Lewis formulas is incorrect?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the ground-state electron configuration of Co3+?
A) [Ar]3d5
B) [Ar]3d44s2
C) [Ar]3d6
D) [Ar]3d64s1
E) [Ar]3d54s2
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of the following species is the octet rule violated by the central atom when the central atom has a formal charge of zero?
A) SOCl2
B) CCl4
C) H2S
D) PF3
E) N2F4
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is true?
A) The triple bond in N2 has a smaller bond order and a smaller bond length than the single bond in F2.
B) The triple bond in N2 has a larger bond order and a smaller bond length than the single bond in F2.
C) The triple bond in N2 has a smaller bond order and a larger bond length than the single bond in F2.
D) The triple bond in N2 has a larger bond order and a larger bond length than the single bond in F2.
E) The triple bond in N2 and the single bond in F2 have the same bond order and the same bond length.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based on the following data,what is the I-I bond energy? 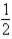 H2(g) +
H2(g) + 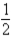 I2(g) → HI(g) ; ΔH = 26.36 kJ
Bond
Bond Energy (kJ/mol)
H-H
435
H-I
295
I2(g) → HI(g) ; ΔH = 26.36 kJ
Bond
Bond Energy (kJ/mol)
H-H
435
H-I
295
A) 365 kJ/mol
B) 208 kJ/mol
C) -208 kJ/mol
D) -155 kJ/mol
E) 155 kJ/mol
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 125
Related Exams